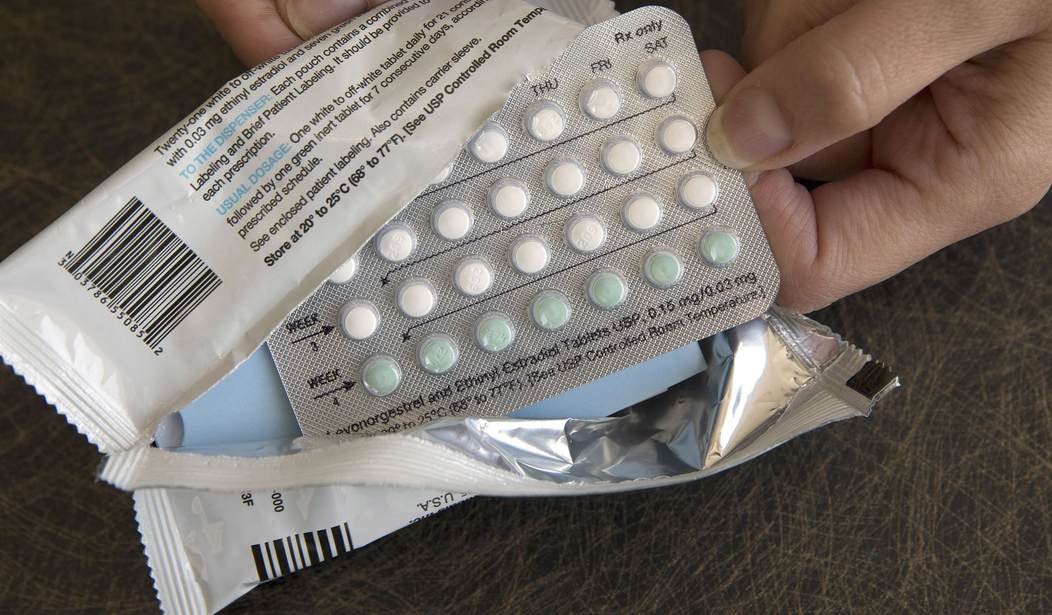
The Food and Drug Administration on Thursday approved the first over-the-counter birth control pill in the U.S.
In early 2024, Perrigo’s once-a-day Opill, a progestin-only pill, will be available at drug stores, supermarkets, and online.
“Today’s approval marks the first time a nonprescription daily oral contraceptive will be an available option for millions of people in the United States,” said Patrizia Cavazzoni, M.D., director of the FDA’s Center for Drug Evaluation and Research, on Thursday. “When used as directed, daily oral contraception is safe and is expected to be more effective than currently available nonprescription contraceptive methods in preventing unintended pregnancy.”
There is no age restriction on the pill, despite concerns over correct usage and possible health hazards associated with it.
Seventeen advisers to the FDA voted unanimously in May to make Opill available over the counter despite concerns the agency raised about data Perrigo submitted with its application, including a study showing that many participants reported taking more pills than they were dispensed. The company said it couldn’t explain the discrepancy.
The agency questioned whether younger people and people with limited literacy could follow the dosing directions and said researchers likely used methods that exaggerated positive results. Perrigo said standards met or exceeded those used in other studies exploring over-the-counter drug adherence. (WSJ)
Kristan Hawkins of Students for Life said the news should mean pro-choice advocates won’t need abortions anymore, according to their own arguments.
I guess everyone who is pro-choice will join me in calling for an end to legal abortion now that any woman, girl, or rapist can get birth control pills over the counter. Right?
— Kristan Hawkins (@KristanHawkins) July 13, 2023
I've heard this time and time again that if we just had more easily accessed birth control, then we…
Recommended
Others pointed out that media reports are not hiding the fact that it was a political decision.
Health experts, citing the pill’s lengthy record of safety and effectiveness, have pushed for a nonprescription pill for years, but their campaign took on new urgency after the Supreme Court last year struck down the fundamental right to abortion established by Roe v. Wade. (WaPo)
Since the Supreme Court overturned the national right to an abortion last year, the accessibility of contraception has become an increasingly urgent issue. (NYT)
Though the drug itself and the campaign for its approval go back decades, pressure on the Biden administration to approve a hormonal over-the-counter birth control option increased after the fall of Roe v. Wade last year. (Politico)
There are dozens of experimental drugs that might well save lives that FDA has held up for years.
— David Harsanyi (@davidharsanyi) July 13, 2023























Join the conversation as a VIP Member