It was one week after the terrorist attacks on 9/11 when envelopes containing a white powder began showing up at random locations in four states; among them, a newspaper office in Florida, the Washington D.C. office of then-Senate Majority Leader Tom Daschle, NBC News and the New York Post.
The white powder turned out to be anthrax spores, engineered to be readily dispersed and inhaled – a potentially deadly bioterrorism weapon.
Anthrax infections are treated with antibiotics. There are two that are most effective; ciprofloxacin and doxycycline.
At that time, I was the CEO of a small pharmaceutical company that represented foreign API manufacturers in the US. We had a large, domestic customer base to which we marketed dozens of anti-infective agents, including antibiotics. Doxycycline was one of them.
We had been working with Zenith Laboratories, a generic pharmaceutical manufacturer in South Florida (currently a part of Teva Pharmaceuticals) to approve our doxycycline for use in its formulations. Normally, the turn-around time for the FDA to approve a drug, even a generic copy of an existing drug (which doxycycline was), is well over a year and often two.
But this was different. The US was facing a crisis in the form of a potential bioterrorism attack.
The federal government’s response to anthrax quickly became a national emergency. Zenith Laboratories, along with other manufacturers was awarded a contract to supply tablets and capsules to the Department of Defense’s Strategic National Stockpile.
In less than one month, the Food and Drug Administration granted an emergency use authorization and we became approved suppliers of doxycycline.
Recommended
Over the ensuing months, our logistical challenge was to supervise the manufacture and delivery of as much API as possible to Zenith Laboratory’s manufacturing sites in the US and Puerto Rico.
When the crisis finally subsided, we had delivered close to 200 metric tons (200,000 Kg) of doxycycline.
Fortunately, anthrax never became the bioterrorism threat many had feared. Five people died as a result of coming into contact with envelopes contaminated with the spores that had been delivered through the postal system.
Our government’s coordinated response in 2001 to apply pressure to drug manufacturers and its own Food and Drug Administration to expedite approval of a life-saving treatment for a bioterrorism weapon bears an eerie similarity to the national health crisis in which we find ourselves.
Yet, I don’t recall President George W. Bush ever coming under attack for pressuring the FDA to rush an approval and putting the safety of the nation at risk.
Any drug, be it an antibiotic, an antiviral, a monoclonal antibody cocktail or a vaccine goes through a rigorous, scientific process long before ever falling into the hands of government regulators, let alone politicians.
Drug development begins with a conceptual design model followed by research, engineering, small-scale manufacturing and several phases of testing; usually first in animals and then humans. Failures are common along every step of this process. By some estimates over 90 percent of drugs never make it to market.
Any drug or therapeutic must demonstrate efficacy and safety before the FDA will approve its use in the general public.
So, what do we know about the efficacy and safety of two of the leading mRNA coronavirus vaccines currently in development by Pfizer and Moderna?
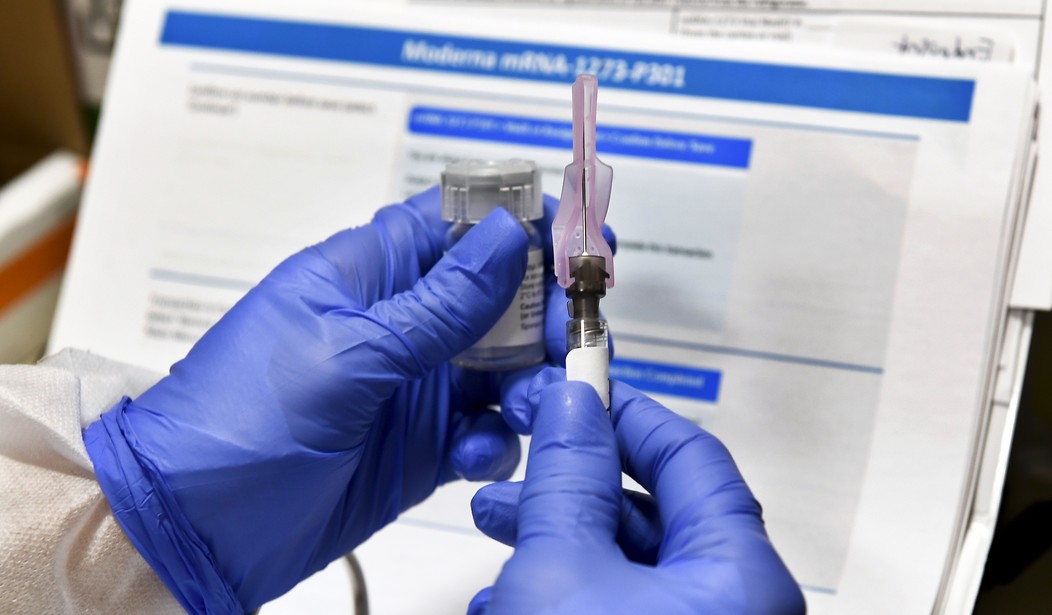
Both companies are well into their Phase 3 clinical trials. Moderna is testing its vaccine on 30,000 people nationwide. In September, Pfizer expanded its Phase 3 cohort to 44,000.
Moderna reported positive results in mid-July from the Phase 1 study of its mRNA-1273 vaccine which “induced anti–SARS-CoV-2 immune responses in all participants, and no trial-limiting safety concerns were identified.”
In August, Pfizer reported similar findings: “7 days after a second dose of 30µg, BNT162b2 elicited SARS-CoV-2–neutralizing geometric mean titers (GMTs) in younger adults (18-55 years of age) that were 3.8 times the GMT of a panel of 38 sera of SARS-CoV-2 convalescent patients, and in older adults (65-85 years of age) the vaccine candidate elicited a neutralizing GMT 1.6 times the GMT of the same panel, demonstrating strong immunogenicity in younger and older adults.”
To date, both vaccines have demonstrated safety and the ability to generate antibodies to the coronavirus at multiples higher than patients that had recovered from an infection.
With any new technology, there is always risk.
During the Manhattan Project when the US tested its first atomic bomb, there was the fear that the explosion would begin a chain reaction and “ignite Earth's atmosphere… destroy[ing] the planet.”
In the name of science, we lost one crew of Apollo astronauts and almost lost a second. We also lost two crews aboard Space Shuttles. There are stories of others who have taken risks to advance science.
In contrast, there have been no reports of death or even grave illness arising among volunteers involved in either the Pfizer or the Moderna mRNA vaccine Phase 3 trials.
Early on, Moderna reported that there were “adverse events… in more than half the participants includ[ing] fatigue, chills, headache, myalgia, and pain at the injection site,” during Phase 1 trials. But these were with the highest dosage, which has since been modified for its ongoing Phase 3 trials.
In late August, Pfizer reported similar positive findings on its second mRNA vaccine candidate, developed to reduce adverse effects from its initial vaccine candidate but providing the same immune response.
It has been more than two months since these trials began. Candidates have by now received their first and second booster injections and have provided blood samples to researchers. I am confident that both companies’ trials will continue to show efficacy and safety.
It is no stretch of the imagination to believe we can and should have coronavirus vaccines approved under the FDA’s emergency authorization use as early as next month for distribution to, at the very least, healthcare workers and those most at risk of severe morbidity.
This is not politics but, in fact, the result of science – lots of science – and shame on those politicians who continue to make this an issue of anything but.
Gregory J. Rummo is a Lecturer of Chemistry at Palm Beach Atlantic University and a Contributing Writer for The Cornwall Alliance for the Stewardship of Creation. He is the former CEO of New Chemic US Inc and patient 001 in Moderna’s mRNA-1273 Phase 3 trial currently being conducted at the Palm Beach Research Center in West Palm Beach, FL. The views expressed in his columns are his own.
























Join the conversation as a VIP Member